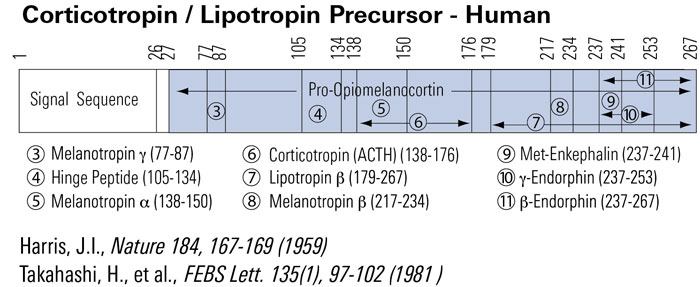
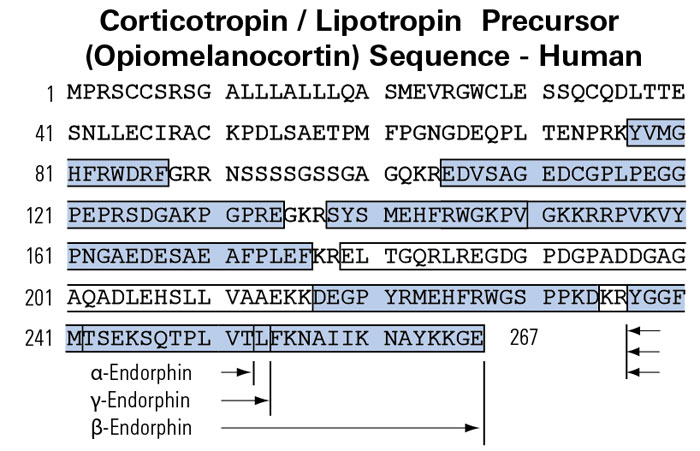
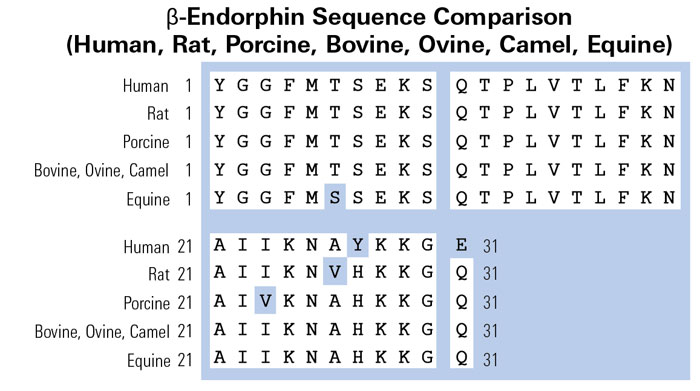
Metformin increases pressure pain threshold in lean women with polycystic ovary syndrome.
BACKGROUND: Despite the strong preclinical rationale, there are only very few data considering the utility of metformin as a potential pain therapeutic in humans. The aim of this study was to determine the association between metformin therapy and pressure pain threshold (PPT) in lean women with polycystic ovary syndrome (PCOS). We hypothesized that metformin therapy in lean PCOS women increases PPT. MATERIALS AND METHODS: Twenty-seven lean PCOS women with free androgen index phenotype >5 and 18 lean healthy controls were enrolled in the study. Fifteen of the PCOS women were randomly assigned to be treated with metformin 1,500 mg daily for 6 months. PPT and plasma β-endorphinlevels were measured in all women at the beginning of the study and after 6 months of observation. RESULTS: We observed an increase in PPT values measured on deltoid and trapezius muscle in the PCOS with metformin group after 6 months of metformin administration (4.81±0.88 kg/cm(2), P<0.001 on deltoid muscle, and 5.71±1.16 kg/cm(2) on trapezius muscle). We did not observe any significant changes in PPT values in the PCOS without treatment group and in controls. We did not observe any significant changes in serum β-endorphinlevels in any studied groups during the 6-month observation. CONCLUSION: We conclude that metformin therapy increases PPT in lean PCOS women, without affecting plasma β-endorphin concentration. Our results may suggest the potential role of metformin in pain therapy. We propose that larger, randomized studies on metformin impact on pain perception should be performed.This publication used a beta-endorphin RIA kit (#RK-022-14) from Phoenix Pharmaceuticals.
Kia?ka M, Milewicz T, Sztefko K, Rogatko I, Majewska R. Drug Des Devel Ther. 2016;10:2483-90.
Effects of tail docking and castration on stress responses in lambs and the influence ofprenatalglucocorticoidtreatment.
It is common practice in Australian agriculture to remove the tails of lambs to prevent infection and to castrate males to prevent behavioural problems and unwanted reproduction. We have studied the pain and stress responses to these interventions by measuring changes in the hypothalamic-pituitary-adrenal (HPA) axis and β-endorphin levels. Further, we have evaluated the effects ofprenatalexposure to dexamethasone, which is known to affect the developing HPA axis. In control animals that had receivedprenatalsalinetreatment, plasma cortisol and adrenocorticotrophin (ACTH) levels increased after the interventions in both females and males. Plasma β-endorphin levels also increased after the interventions, but the responses were less consistent.Prenataldexamethasone exposure early in pregnancy (dexamethasone 0.14 mg kg(-1) ewe weight injection commenced on day 40 of pregnancy for four consecutive intramuscular injections at 12-hourly intervals) blunted the cortisol response to tail docking in female offspring, but not to combined tail docking and castration in males. It had no effect on ACTH or β-endorphin responses in either sex. These findings describe the stress responses to these common agricultural interventions and suggest that long-term development of the HPA axis in females is altered byprenatalexposure to dexamethasone.This publication used a beta-endorphin RIA kit (#RK-022-14) from Phoenix Pharmaceuticals to measure plasma levels.
Li S, Nitsos I, Polglase GR, Newnham JP, Challis JR, Moss TJ. Reprod Fertil Dev. 2013;25(7):1020-5.
Is beta endorphinrelatedtomigraineheadacheand itsrelief?
BACKGROUND: Lowβendorphinlevel in serum and cerebrospinal fluid (CSF) has been reported inmigraine. The basis of painreliefinmigraineby repetitive transcranial magnetic stimulation (rTMS) may berelatedto βendorphin(BE), which has not been evaluated. It is proposed to measure plasma βendorphinlevel inmigrainepatients and the change inβendorphinlevel following rTMS, and to correlate these changes withmigrainerelief. METHODS: Twenty-five patients withmigrainediagnosed as per InternationalHeadacheSociety criteria and 20 gender- and age-matched controls were included. Their clinical characteristics including duration ofmigraine, its frequency, severity and functional disability, triggers, allodynia and number of analgesic used were noted. Plasma βendorphinlevel was estimated before and after the third rTMS session. rTMS was delivered on the hot spot of right abductor digiti minimi on alternate days for 3 days and each session consisted of 600 pulses at 10 Hz. The clinical response was noted weekly for 1 month and correlated with βendorphinlevel. RESULTS: The median age of the patients was 35 (20-50) years and 19 were females. Eight patients had episodic and 17 chronicmigraine. βendorphinlevel was significantly lower inmigraine(4.35 ± 2.29 ng/ml) compared to controls (6.68 ± 2.93 ng/ml). βendorphinlevel was lower in chronic compared to episodicmigraine(3.74 ± 2.20 versus 5.65 ± 2.02 ng/ml). Following rTMS, theheadachefrequency, severity, functional disability and analgesic intake significantly reduced on the seventh day of rTMS and remained significant until the fourth week compared to the baseline. The clinical improvement was associated with increase inβendorphinlevel (4.35 ± 2.29 versus 6.58 ± 3.33 ng/ml). CONCLUSION: It can be concluded from this study that the basal plasmaβ endorphinlevel was low inmigrainepatients, especially in chronicmigraine. The improvement inmigraineafter rTMS was associated with increase in βendorphinlevel.This publication used a beta-endorphin EIA kit (#EK-022-14) from Phoenix Pharmaceuticals.
Misra UK, Kalita J, Tripathi GM, Bhoi SK. Cephalalgia. 2013;33(5):316-22.
Possible involvement of beta-endorphin in docosahexaenoic acid-induced antinociception
We have previously demonstrated that then-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) has an antinociceptive effect on various pain stimuli in a naloxone-reversible manner. In the present study, the role of the endogenous opioid peptide β-endorphin in DHA-induced antinociception was examined. DHA-induced antinociception was abolished when mice were pretreated with the δ-opioid receptor antagonist β-funaltrexamine (β-FNA) and the δ-opioid receptor antagonist naltrindole, but not by the δ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) in the acetic acid-induced writhing test. In the radioligand binding assay, DHA itself did not have affinity for μ- , δ- or κ-opioid receptors. On the other hand, the pretreatment of anti-β-endorphin antiserum inhibited DHA-induced antinociception. Furthermore, the intracerebroventricular injection of DHA dose-dependently reduced writhing behavior, and this effect was inhibited byd-Phe-Cys-Tyr-Orn-Thr-Pen-Thr-NH2(CTOP) and naltrindole, but not nor-BNI. β-endorphin-induced antinociception was inhibited by the pretreatment of β-FNA, but not naltrindole or nor-BNI, and its levels in plasma were increased by DHA treatment. These findings suggest that the induction of antinociception by DHA may partially involve the δ-opioid receptor via the release of β-endorphin.This publication used a beta-endorphin EIA kit (#EK-022-06) from Phoenix Pharmaceuticals.
Nakamoto K, Nishinaka T, Ambo A, Mankura M, Kasuya F, Tokuyama S. Eur J Pharmacol. 2011;666(1-3):100-4.
Nitrousoxide-inducedNO-dependentneuronalreleaseof beta-endorphin from theratarcuatenucleusand periaqueductal gray.
Nitrous oxide (N(2)O)-induced antinociception is thought to result from nitric oxide (NO)-dependent neuronal release of endogenous opioid peptides in the central nervous system. The present study employed microdialysis to determine whether exposure to N(2)O stimulates proopiomelanocortin (POMC) neurons to release β-endorphin in the arcuate nucleus (ARC) of the hypothalamus and the periaqueductal gray (PAG) of the midbrain. Male Sprague-Dawley rats were stereotaxically implanted with microdialysis probes in the ARC or PAG. Exposure to 70% N(2)O significantly increased dialysate levels of oxidation products of NO as well as β-endorphin, compared to levels in fractions collected under room air. These increases in the ARC and PAG were abolished by systemic pretreatment with L-N(G)-nitro arginine methyl ester (L-NAME). These findings suggest an association between increased NO activity and the stimulated release of β-endorphin during exposure of rats to N(2)O.This publication used a beta-endorphin EIA kit (#EK-022-06) from Phoenix Pharmaceuticals.
Ohgami Y, Chung E, Quock RM. Brain Res. 2010;1366:38-43.
SUBSTANCE P AND BETA ENDORPHIN MEDIATE ELECTROACUPUNCTURE INDUCED ANALGESIC ACTIVITY IN MOUSE CANCER PAIN MODEL.
Cancer pain impairs the quality of life of cancer patients, but opioid analgesics can not only cause inhibition of respiratory function, and constipation, but also other significant side effects such as addiction and tolerance that further decrease quality of life. Thus, in the present study, the effects of electro-acupuncture treatment (EA) on mechanical allodynia were examined in cancer pain mouse model. In order to induce neuropathic cancer pain model, S-180 sarcoma cells were inoculated around the sciatic nerve of left legs of Balb/c mice. The mass of S-180 cancer cells embedded around sciatic nerve in a time course was confirmed by Magnetic Resonance Imaging (MRI) scanning. Mechanical allodynia was most consistently induced in mouse sarcoma cell line S-180 (2 x 10(6) sarcoma cells) treated group among all groups. EA stimulation (2Hz) was daily given to ST36 (Zusanli) of S-180 bearing mice for 30 min for 9 days after S-180 inoculation. EA treatment significantly prolonged paw withdrawal latency from 5 days after inoculation as well as shortened cumulative lifting duration from 7 days after inoculation compared with tumor control. In addition, the overexpressions of pain peptide substance P in dorsal horn of spinal cord were significantly decreased in EA treated group compared with tumor control on Day 9 after inoculation. Furthermore, EA treatment effectively increased the concentration of beta endorphin in blood and brain of mice more than tumor control as well as normal group. The concentration of beta-endorphin for EA treatment group increased by 51.457% in blood 12.6% in brain respectively, compared with tumor control group. These findings suggest that S-180 cancer pain model can be a consistent and short time animal model and also EA treatment can be an alternative therapeutic method for cancer pain via decreased substance P and increased beta endorphin. This publication used a beta-endorphin EIA kit (#EK-022-06) from Phoenix Pharmaceuticals.
Lee HJ, Lee JH, Lee EO, et al. Acupunct Electrother Res. 2009;34(1-2):27-40.
HUMAN PITUITARY CONTAINS DUAL CATHEPSIN L AND PROHORMONE CONVERTASE PROCESSING PATHWAY COMPONENTS INVOLVED IN CONVERTING POMC INTO THE PEPTIDE HORMONES ACTH, ALPHA-MSH, AND BETA-ENDORPHIN.
The production of the peptide hormones ACTH, alpha-MSH, and beta-endorphin requires proteolytic processing of POMC which is hypothesized to utilize dual cysteine- and subtilisin-like protease pathways, consisting of the secretory vesicle cathepsin L pathway and the well-known subtilisin-like prohormone convertase (PC) pathway. To gain knowledge of these protease components in human pituitary where POMC-derived peptide hormones are produced, this study investigated the presence of these protease pathway components in human pituitary. With respect to the cathepsin L pathway, human pituitary contained cathepsin L of 27-29 kDa and aminopeptidase B of approximately 64 kDa, similar to those in secretory vesicles of related neuroendocrine tissues. The serpin inhibitor endopin 2, a selective inhibitor of cathepsin L, was also present. With respect to the PC pathway, human pituitary expresses PC1/3 and PC2 of approximately 60-65 kDa, which represent active PC1/3 and PC2; peptide hormone production then utilizes carboxypeptidase E (CPE) which is present as a protein of approximately 55 kDa. Analyses of POMC products in human pituitary showed that they resemble those in mouse pituitary which utilizes cathepsin L and PC2 for POMC processing. These findings suggest that human pituitary may utilize the cathepsin L and prohormone convertase pathways for producing POMC-derived peptide hormones. Hook V, Funkelstein L, Toneff T, Mosier C, Hwang SR. Endocrine. 2009;35(3):429-37.
CHANGES IN SERUM LEPTIN AND BETA ENDORPHIN LEVELS WITH WEIGHT LOSS BY ELECTROACUPUNCTURE AND DIET RESTRICTION IN OBESITY TREATMENT.
This study aims to investigate the role of changes in leptin and beta endorphin (BE) levels in weight loss following electroacupuncture (EA) application in obesity treatment. EA was applied to 20 females who were 41.45 +/- 4.71 years old and had a body mass index of 36.00 +/- 2.66; and a diet program was applied to 20 females who were 42.30 +/- 4.35 years old and had a body mass index of 34.90 +/- 3.21. There was a 4.5% weight reduction in the patients with EA application, whereas patients on diet restriction had a 3.1% weight reduction. A decrease of loss of body weight was observed in the EA group (p < 0.000) when compared against the diet restricted group. A decrease of serum leptinlevels (p < 0.000) and an increase in the serum BE (p < 0.05) levels were observed in the EA group compared to the diet restricted group. In this study, reduced serum leptin levels paralleling to weight loss were observed in the EA group. Furthermore, it is thought that in the EA applied group, increasing serum BE level probably enhanced the lipolitic activity which may have caused weight loss in obese people by mobilizing energy stores. It may be considered that the EA application with diet restriction in obesity treatment is more effective than the dietrestriction alone. This publication used a beta-endorphin EIA kit (#EK-022-06) from Phoenix Pharmaceuticals.
Cabioglu MT, Ergene N. Am J Chin Med. 2006;34(1):1-11.